Fully customized automated test solutions designed specifically for medical device electronics, including R&D and production testing, in order to meet global compliance requirements
The purpose of safety testing medical electronic equipment is to be sure that a device is safe from electrical hazards to the patient and caregivers. There are a number of UL, European and Canadian standards that serve as the ruling body on how medical products will be tested, one in particular, IEC60601 (the International Electrical Safety Standard for medical electronic equipment) is experiencing “global harmonization,” meaning it has been accepted and implemented around the world. The IEC60601 standard is mainly intended for product development where safety considerations must be taken into account early in the design phase of a product, however much of it is applied to production line testing since it is the only way for a manufacturer to be sure they are shipping safe product.
Sentinel III – Medical System
Chroma Sentinel III
- Chroma 19032, CaptivATE, 1500VA or 800VA AC Source, Matrix 8000 Scanning System
- Testing to IEC 60601-1 & IEC 60601-2-49
- F-Type LC (Mains-on-Applied-Part)
- Multi-Point Hipot, GB and LC Testing
- Up to 16 Patient (Applied Part) Connections
- Power-Up Computerized Devices
Sentinel Series: IEC60601 Medical Device Automated Test Systems with IQ/OQ Test Protocol Documents
Sentinel systems are available in 3 efficient configurations with or without an AC power source to fit a wide variety applications from 1 to multiple patient connections. CaptivATE software simplifies testing by accurately automating all testing and outputting all test results including IQ/OQ documentation. All systems include tests for ground bond, AC and DC Hipot, insulation resistance, and leakage measurements. Advanced systems include our programmable AC power sources and Matrix 8000 scanners for multi-point patient connections and increased test capabilities.
CaptivATE Automation Software
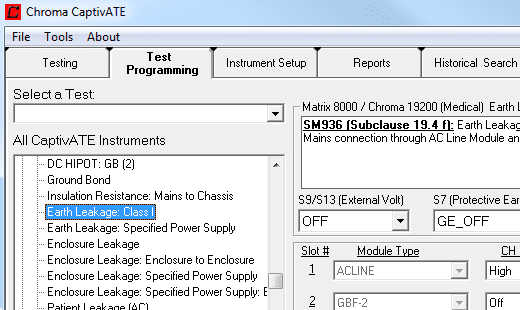
CaptivATE provides an off-the-shelf test platform for automating hipot, leakage current, and functional tests. By automating the loading of test setups, sharing test setups plantwide, and combining safety and functional tests into one system, CaptivATE increases efficiency and productivity. For medical devices, software supports IEC60601-1 test requirements, digitally stores product test certificates, and provides IQOQ protocol documentation.



